جدول المحتويات
Martensite is a special crystalline structure formed in tool steel during the hardening process. This structure is named after the renowned German metallurgist Adolf Martens, who first discovered it in 1878. If the purity of the raw material or the forging ratio is insufficient, even subsequent heat treatment may fail to produce a perfect martensite structure. This can lead to chipping or insufficient wear resistance in molds and tools.
The Nature and Formation of Martensite
Martensite is not a stable structure that forms naturally during the slow cooling of tool steel after heating; it is a metastable phase. During conventional slow cooling, الأوستينيت in the steel microstructure decomposes into ferrite and cementite (pearlite) through atomic diffusion. The formation of martensite is entirely different. When we rapidly cool tool steel heated to high temperatures—a process known as quenching—the diffusion movement of atoms within the metal is forcibly suppressed. Atoms lack the time to diffuse, forcing structural transformations to occur through a collective, coordinated “shearing” motion.
Essentially, martensite is a supersaturated solid solution of carbon in α-iron. Quenching traps carbon atoms in the crystal lattice’s interstitial sites. This results in a body-centered cubic (BCC) structure within the steel’s microstructure. Trapped carbon atoms stretch the lattice in one direction (the c-axis), transforming the crystal structure into a body-centered tetragonal (BCT) structure and causing significant lattice distortion. This immense internal stress at the microscopic level is precisely the fundamental reason why mold steels such as د2 أو ح13 achieve ultra-high strength and hardness after quenching. The higher the carbon content, the more severe the lattice distortion, and the greater the hardness. Throughout this process, the chemical composition of the martensite remains unchanged.
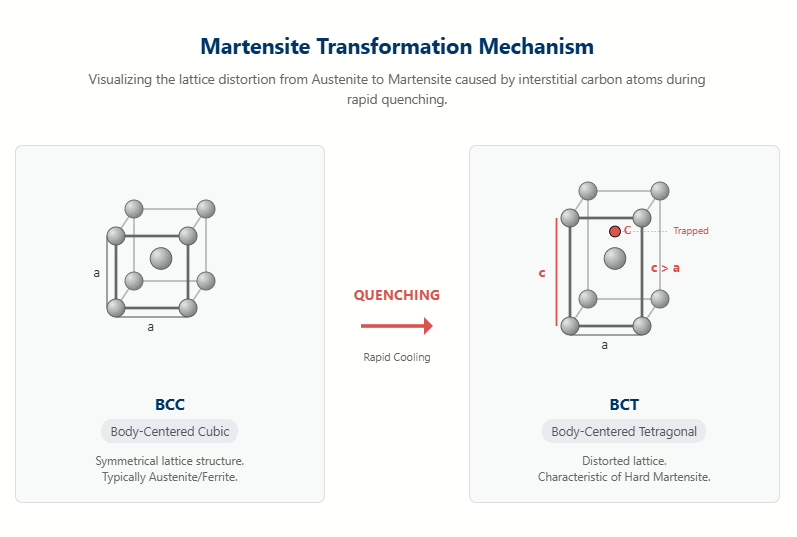
في heat treatment of tool steel, two critical temperature points exist: the Mس point—the start temperature of the martensitic transformation—and the Mf point—the end temperature of the martensitic transformation. When steel cools to the Mس temperature, the martensitic transformation begins instantaneously. The steel’s chemical composition primarily determines this temperature. Carbon and manganese are the most significant elements in lowering the Mس point. The martensitic transformation is considered substantially complete only when the temperature falls below the Mf point. Typically, the Mf point is approximately 200°C lower than the Mس نقطة.
This leads to a common quality issue in tool steels—retained austenite. D2, a high-alloy steel, has both very low Mس and Mf points, with Mf even below room temperature. If the cooling temperature during heat treatment fails to reach Mf, untransformed austenite remains in the steel. Retained austenite causes insufficient hardness, dimensional instability, and even deformation during use in molds and tools. This is why D2 steel often requires cryogenic treatment, which lowers the temperature below the Mf point to eliminate retained austenite.
Martensite Morphology
In tool steels, not all martensite is the same. Depending on the steel’s carbon content, martensite exhibits two distinct forms.
1. Lath Martensite
Its microstructure resembles bundles of parallel, slender wooden planks forming discrete “packets.” This structure is internally saturated with high-density dislocations. Although hard, it retains excellent “slip capability.” This structure is commonly found in low-carbon and medium-carbon steels, such as our H13 (1.2344) hot-work tool steel. Due to the lamellar martensite, H13 (1.2344) steel exhibits excellent toughness, enabling it to withstand thermal shock without cracking.
2. Plate Martensite
This form of martensite exhibits lenticular or sharply needle-like structures. Unlike Lath Martensite structures, these are not neatly aligned but instead distributed non-parallel and interlocked, making slip extremely difficult. Consequently, it exhibits exceptionally high hardness and wear resistance, but with poor toughness. This structure is commonly found in high-carbon steels, such as our D2 (1.2379) and د3 (1.2080) cold-work tool steels.
Mechanical Properties and Thermal Treatment
Martensite is the microstructure with the highest hardness and strength in steel. When the carbon content of tool steel reaches approximately 0.60%, the steel’s quenched hardness peaks at around 68 HRC. Further increases in carbon content, such as D2 steel, do not significantly enhance the hardness of the martensitic matrix. The excess carbon is primarily utilized to form wear-resistant carbides. Fresh martensite in the “as-quenched” state is extremely hard but highly brittle, with low fracture resistance. Without subsequent processing, it is fundamentally unsuitable for industrial applications.
To resolve brittleness issues, nearly all quenched steels must undergo tempering. Tempering is a diffusion process in which carbon atoms move within the microstructure. The supersaturated carbon atoms, once trapped within the crystal lattice, have the opportunity to escape. Low-temperature tempering precipitates fine ɛ(epsilon) carbides, relieving some residual stresses. High-temperature tempering promotes the precipitation of more stable carbides or alloy carbides. In summary, tempered steel becomes slightly softer, while toughness and ductility are significantly enhanced, and residual internal stresses are released. This refined microstructure is referred to as tempered martensite.
Some of our customers who produce molds for slender rods or complex shapes frequently encounter issues with heat treatment distortion or cracking. We may recommend martempering to them. During quenching, do not cool directly to the final temperature. Instead, hold the temperature slightly above the Mس point (martensite start transformation temperature) until the surface temperature and core temperature of the workpiece equalize. Once internal and external temperatures are uniform, continue cooling. This method significantly reduces thermal stress, minimizing the risk of deformation and cracking.
Closing Remarks
As Aobo Steel’s primary product is tool steel, we interpret martensite from a tool-steel perspective. However, from a materials science standpoint, the martensitic phenomenon is actually quite widespread and is not exclusive to iron-carbon alloys. Similar martensitic phase transformations occur in numerous non-ferrous metals—such as copper, titanium, and nickel alloys—and even in ceramics and minerals. Martensite in non-ferrous metals may not exhibit the same hardness as in steel; some variants are soft or ductile. Take nickel-titanium alloys, for instance: their remarkable shape-memory capability—where they automatically return to their original form after bending—is fundamentally based on a specific martensitic transformation mechanism.
